Clinical trials

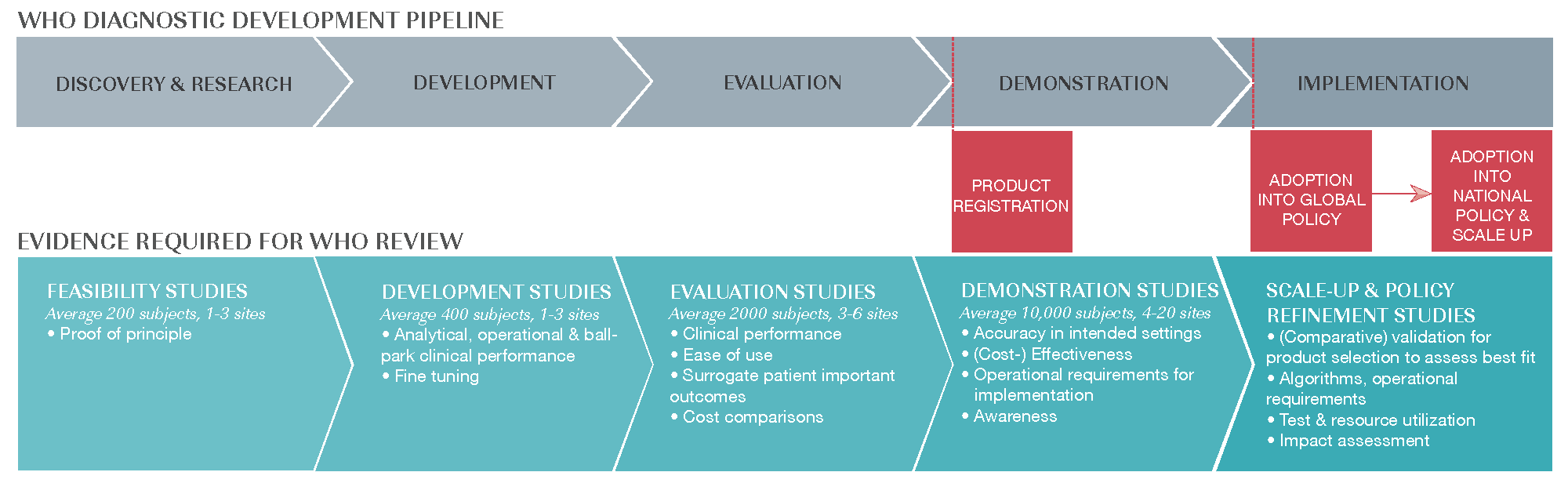
Our well-established clinical trial unit enables the collection of essential evidence for researchers, developers, and implementers of new diagnostics. Our team designs and conducts feasibility, development, evaluation and demonstration studies.
We help to build capacity and strengthen health systems in low-resource settings, supporting the development of in-country expertise so that clinical trials meet international standards. We also gather evidence for scale-up and policy refinement after products have received WHO approval and been adopted into global policy.
Since 2015, over 32,500 patients have been enrolled in FIND-supported trials, over 6,000 healthcare workers have been trained and over 3,000 laboratories and testing sites have been strengthened. Our network of clinical trial partners spans 19 low- and middle-income countries.
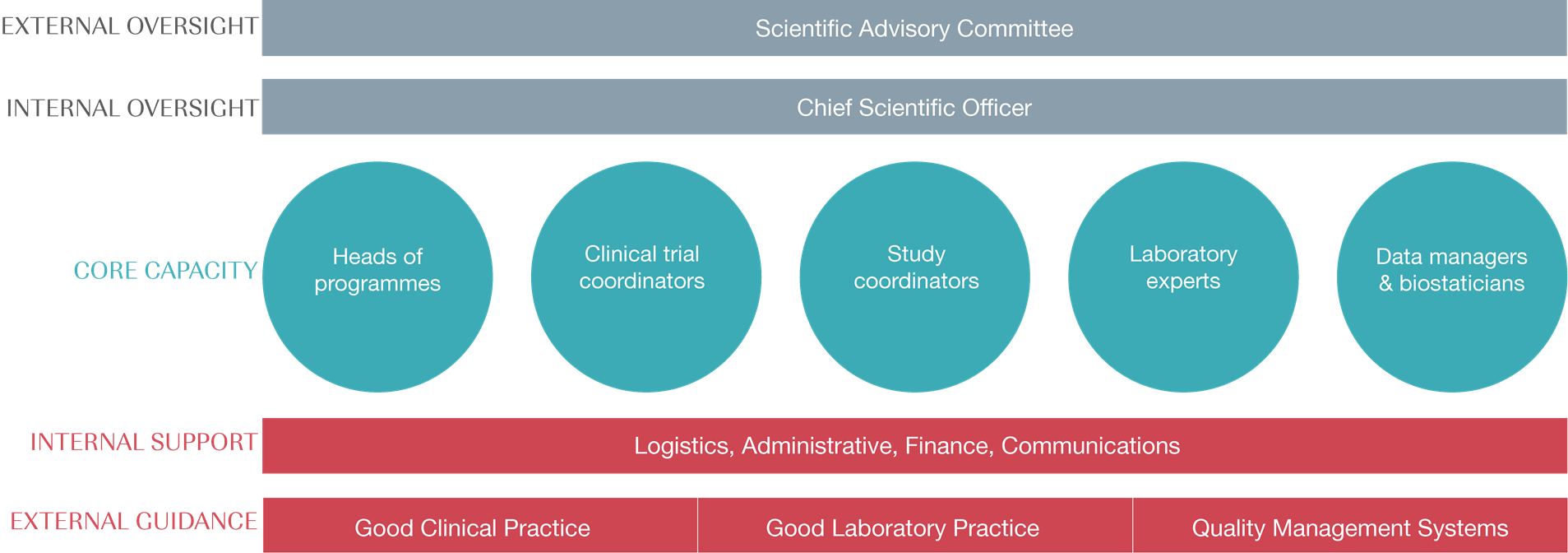
Our clinical trial unit benefits from internal oversight from our Chief Scientific Officer with support from our Head of Clinical Trials & Regulatory Affairs, and external oversight from our Scientific Advisory Committee.
FIND is an Enterprise Member of the Regulatory Affairs Professionals Society (RAPS).
Clinical pathway to WHO approval
Quick links